By Alexandra Demetriou
The Argus II retinal prosthesis––often referred to as the world’s first “bionic eye” ––revolutionized the treatment of blindness. Throughout the past decade, this artificial retina, developed by Mark Humayun, MD, PhD and colleagues in collaboration with Second Sight Medical Products, has enabled hundreds of patients around the world with complete retinal blindness to regain partial eyesight.

The invention currently allows for black-and-white vision and particularly helps patients detect boundaries between objects in their surroundings. Using a combination of patient feedback and laboratory experiments, Humayun and his team at the USC Dr. Allen and Charlotte Ginsburg Institute for Biomedical Therapeutics continue to enhance the visual information retinal prostheses can offer patients.
In their latest work, USC Ginsburg Institute scientists, in collaboration with USC Institute for Technology and Medical Systems (ITEMS) engineers, have developed detailed computer models of the retina that enable them to test modifications to the prosthetic system with unprecedented ease and speed. These advances in computational modeling have enabled the team to make profound strides forward in understanding how to refine and build upon the device’s capabilities––with the long-term goal of restoring color vision and more precise visual details to patients with progressive blindness.
A look inside the “bionic eye”
At the most basic level, retinal prostheses work by converting visual information from the environment into electrical impulses that stimulate the otherwise blind retina. When these signals reach the vision center of the brain, known as the visual cortex, patients are able to perceive images and shapes from their surroundings.
The Argus II system involves a retinal implant––or, more specifically, a microelectrode array that sits atop and stimulates the retina––coupled with a pair of glasses outfitted with a camera that transmits signals to the implant. When the implant receives visual cues from the camera, it generates small electrical impulses that stimulate the patient’s retinal cells. This stimulation causes the patient to perceive a flash of light that vision scientists refer to as a phosphene.

Phosphenes should ideally appear as small, rounded dots when a single electrode stimulates the retina. In practice, however, patients sometimes perceive a dash instead of a dot––and the reason has to do with the way cells intermesh in the retina.
One of these cell types is the retinal ganglion cell, which plays an essential role in sending visual information from the eye to the brain. Retinal ganglion cells have rounded bodies with many thin projections branching off in different directions and one long projection that feeds information to the brain. These projections overlap and intertwine like the branches of trees in a densely wooded forest, so that when an electrode stimulates one of the retinal cells it often activates the projections of neighboring cells as well.
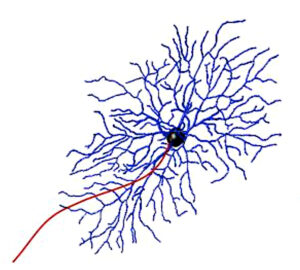
When electrical signals from the prosthesis stimulate both cell bodies and projections from nearby cells, patients perceive a dash of light, called an elongated phosphene, instead of a discrete dot. Humayun’s team set out to determine how to prevent elongated phosphenes from forming so patients could enjoy more refined, detailed vision. Doing so required devising an approach to target retinal cell bodies without activating neighboring cells in the process.
Computer models probe deeper into cellular behavior
To solve this challenge, the USC Ginsburg Institute and USC ITEMS teams first developed computational models to recreate different types of retinal ganglion cells and the patterns in which they overlap. Their models accounted for the relevant functional properties of retinal ganglion cells and could therefore respond to stimuli in ways that mimicked how real retinal ganglion cells would respond. Using these model systems, the scientists ran through a gamut of simulations to find a stimulation pattern that activated cell bodies while avoiding the large projections, known as axons, that other cells use to send visual information to the brain.
In one recent study, the team reported their discovery that very short-duration electrical pulses, as brief as 0.1 milliseconds, can selectively target the intended retinal ganglion cells’ bodies without activating their axons. Their models also enabled them to more accurately map out patterns of cellular activation that arise when shorter pulses are used to stimulate the retina. The researchers hope these, and future, findings can help resolve the problem of elongated phosphenes to ultimately offer patients enhanced visual resolution with a retinal prosthesis.
USC Ginsburg Institute and USC ITEMS scientists are also currently using their computational models of the retina to study possible ways of programming additional visual components, such as color, into the Argus II system. A 2020 study of theirs showed that a particular subtype of retinal ganglion cell, called a bistratified cell, is more responsive to high frequency stimulation than one of its counterparts known as a monostratified cell. Understanding these types of slight differences in responses may help the team improve the performance of retinal prosthetics to enable patients to detect color, contrast and edges of objects. Such research is still ongoing, but based on their preliminary findings, the team is hopeful for the ways they can continue enhancing visual perception for the hundreds of retinal prosthesis users who once lived in complete darkness.
Javad Paknahad, a PhD candidate at USC and the lead author on the aforementioned studies, emphasized how the “multi-scale” nature of these models, which account for the properties of single cells as well as the behavior of entire cell populations, makes this approach particularly valuable. He added that using computer models to study the retina allows their team to run experiments more efficiently, which ultimately accelerates scientific progress. “These multi-scale computational models end up saving a lot of time when doing experiments, because as you continue refining the model system and designing the right programs, you can more readily determine the best approaches to solving complex problems,” he said. “This makes computational modeling very powerful.”
Disclosure: Mark Humayun, MD, PhD, is a co-inventor of the Argus implant series. He is a minority equity owner in Second Sight Medical Products, Inc. and receives royalty payment.
Disclosures: Regenerative Patch Technologies LLC was founded by Mark Humayun, MD, PhD, and David R. Hinton, MD, from the University of Southern California, and Dennis O. Clegg, PhD, from the University of California, Santa Barbara. The technology to produce the stem cell–based retinal implant is exclusively licensed to Regenerative Patch Technologies LLC from the University of Southern California, the California Institute of Technology and the University of California, Santa Barbara. Humayun has an equity interest in and is a consultant for Regenerative Patch Technologies LLC.



