Azad M. Madni, Ph.D., NAE
University Professor
GIBT Affiliate
Under a medical research award from the Marcus Foundation, the CPCB-RPE1 retinal implant is being developed by Dr. Mark Humayun and his team for patients with geographic atrophy, a form of advanced macular degeneration. The Marcus Foundation funds and partners with organizations that are making a difference in the delivery of community food resources or are expanding the understanding and implementation of sustainability. Bernie Marcus of the Marcus Foundation is one of the three founders of Home Depot.
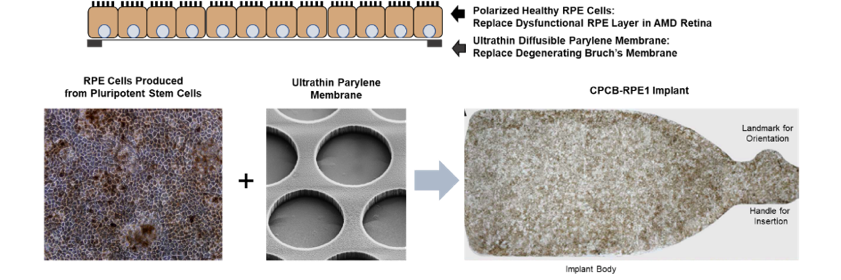
Geographic atrophy is a condition which inevitably progresses to severely impaired vision and ultimately legal blindness. Unlike other approaches including the recent FDA approved injection of medications from the company Apellis, these approaches focus on slowing the progression of the disease and often vision continues to deteriorate. The CPCB-RPE1 implant (see Figure 1) currently under development by Dr Humayun’s team and their collaborators is a unique, first-in-field product that is specifically focused on improving the vision of patients with GA.
Clinical trials of the implant are expected to start in the next year at multiple sites in subjects who have GA. Patients receiving the implant will be evaluated using FDA-cleared safety and efficacy endpoints. Pursuant to demonstrating implant safety and efficacy, randomized clinical trials for the registration of the implant will commence with the goal to obtain FDA approval. In keeping with a systems thinking mindset, both patient and healthcare considerations have been addressed and appropriate measures have been built into the product to facilitate widescale adoption within both the ophthalmologist and patient communities. For example, the surgical implementation procedure has been designed to exploit techniques such as vitrectomy and retinotomy commonly used by retinal surgeons. Along the same lines, a custom-designed delivery tool has been developed and successfully implemented to facilitate targeted delivery of the implant to the geographic area, while ensuring that the implant is protected from damage during the procedure. As important, to facilitate widespread distribution, a cryopreserved formulation of the implant has been developed to ensure product shelf-life longevity thereby vastly simplifying scheduling, delivery and shipment of the implant. Also, the use of long-term storage solutions makes scaled manufacturing of the implant practical. Furthermore, in the light of ongoing work by the Advanced Regenerative Medicine Institute (ARMI), a non-profit primarily funded by the Defense Department, future automation of the manufacturing process is entirely feasible to fulfill the growing market demand at significantly reduced cost. Finally, the cost of the implant has been a driver throughout development. At today’s manufacturing scale, an implant is expected to cost approximately $10,000. Using a conservative two-fold to five-fold reduction in costs with scale-up and manufacturing, the cost of the implant could potentially be reduced to $2k to $5k.
In sum, the CPCG-RPE1 implant is a potential “game-changer.” It is being designed to be affordable while satisfying the patient’s unmet need for which there are no approved therapies to-date.