Synethetic Cellular Scaffolds for Stem Cell Treatment for AMD
 Mark Humayun, MD, PhD and David Hinton, MD, were awarded a $38 million grant from the California Institute for Regenerative Medicine (CIRM) to develop a stem cell therapy for age-related macular degeneration (AMD). Through a cross-disciplinary approach, both Principal Investigators assembled a team of world experts comprised of four major schools and institutions: University of Southern California Eye Institute (USC Roski Eye Institute), University of California-Santa Barbara, California Institute of Technology and City of Hope.
Mark Humayun, MD, PhD and David Hinton, MD, were awarded a $38 million grant from the California Institute for Regenerative Medicine (CIRM) to develop a stem cell therapy for age-related macular degeneration (AMD). Through a cross-disciplinary approach, both Principal Investigators assembled a team of world experts comprised of four major schools and institutions: University of Southern California Eye Institute (USC Roski Eye Institute), University of California-Santa Barbara, California Institute of Technology and City of Hope.
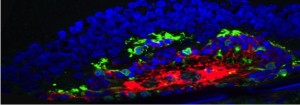
AMD is the nation’s leading cause of blindness in the elderly, with over 2 million who have gone blind and over 250,000 who are going blind each year. AMD affects central vision where individuals are unable to focus straight ahead or even drive.The disease is characterized by a loss of retinal pigment epithelial (RPE) cells, which are located in the back of the eye known as the retina. The loss of RPE are localized to a central region of the retina known as the macula. A novel stem cell-based treatment, CPCB-RPE1, has been developed for patients with dry age-related macular degeneration (AMD), particularly those with geographic atrophy (GA), the advanced form of dry AMD. In the normal retina, the light sensing part of the eye, the photoreceptor cells responsible for vision are supported metabolically and structurally by retinal pigmented epithelial (RPE) cells. Geographic atrophy is characterized by dysfunction and loss of RPE cells, followed by photoreceptor loss. Loss of RPE cells is believed to be a critical contributor to photoreceptor loss and decay of vision.
This novel stem cell treatment merges medicine and engineering, taking regenerated RPE and seeding them onto a synthetic membrane, which could then be placed underneath the diseased portion of the retina. The implanted scaffold of RPE are localized and can function to support and replenish photoreceptors of the retina, which would help restore and prevent vision loss in patients with AMD. Figure A and B below, show the stem cell derived RPE and bioengineered scaffold, respectively. Preclinical and human studies support the premise that replacing dead or dying RPE cells in dry AMD could be a way to slow the disease process, slow vision loss and even improve vision. CPCB-RPE1 treatment is composed of human embryonic stem cell (hESC) derived RPE cells that are seeded onto an ultrathin parylene membrane with diffusion properties similar to that of the native Bruch’s membrane of the retina. Similar to a native eye, the implant provides a single monolayer of RPE cells on a permeable membrane support to restore nutrient and 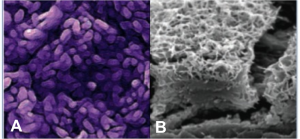 support functions provided by RPE that have deteriorated in patients with advanced AMD.
support functions provided by RPE that have deteriorated in patients with advanced AMD.
The implant is delivered underneath the retina facilitated by a custom delivery tool. Preclinical safety and efficacy studies have shown that CPCB-RPE1 survives in the subretinal space, promotes photoreceptor survival, and improves visual behavior in preclincal models. This unique stem cell therapy was implanted in vivo by Biju Thomas, PhD, assistant professor of research in ophthalmology. Safety and efficacy of the implant was confirmed based on the preclinical studies. As well, results indicated that the stem cell therapy successfully decreased the progression of retinal degeneration in rats. In head-to-head preclinical studies, the CPCB-RPE1 implants showed superiority to single cell RPE suspensions in cell survival, biological function and improvements in visual acuity. Together, these data provide valuable proof-of-concept data to support the development of CPCB-RPE1 for geographic atrophy, a condition that today has poor prognosis, tremendous quality of life impact, and no available treatment options. In this ongoing phase I/IIa clinical trial we are assessing the feasibility of delivery and safety of CPCB-RPE1.
These findings are under evaluation by the FDA in a Phase I/IIa clinical trial in humans. The RPE seeded membranes are manufactured by City of Hope for the clinical trial. The Phase 1 clinical trial, led by Dr. Amir Kashani has begun. The study will include two cohorts of patients. For the first cohort, the study population will be patients with advanced, dry AMD with evidence of significant geographic atrophy. As the safety and tolerability of CPCB-RPE1 is demonstrated in the first cohort, patients with less advanced disease will be recruited into a second cohort in this Phase I/IIa clinical trial and assessed accordingly. For more information please visit https://clinicaltrials.gov/ct2/show/NCT02590692?term=AMD+stem+cell&rank=28. To find out more about the clinical trial at USC please call at 323-865-6935.
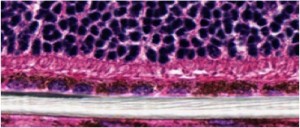 RPE seeded scaffold (white) implanted in the subretinal space of a rat.
RPE seeded scaffold (white) implanted in the subretinal space of a rat.
Novel Bio-scaffold for trauma
Beyond addressing neursensory disorders, IBT is also developing biomaterials-based solutions to prevent neurosensory degradation in the eye caused by trauma. Penetrating injuries to the eye can lead to retinal detachment and blindness if left untreated for days. IBT is currently collaborating with industry and U.S. Army to develop different “patch” technologies to temporarily seal penetrations of the cornea or sclera. These biomaterials may give patients time to see a specialist without risk of vision loss (Jack Whalen, PhD).
Next, read about Neurophotonics.