 Neurosensory disorders of the brain and eye affect a growing number of people. The etiology of these disorders spans a spectrum of factors, which include aging, genetic predisposition, exposure to harmful materials, unhealthy diet, physical injuries and underlying diseases. Physical injuries such as blunt force trauma can activate acute inflammatory responses that can lead to chronic inflammation. This highlights the need for effective therapies for many of these important diseases. Despite this urgent need for treatment of these types of diseases, the lack of effective therapy continues to be one of the greatest health challenges of our time.
Neurosensory disorders of the brain and eye affect a growing number of people. The etiology of these disorders spans a spectrum of factors, which include aging, genetic predisposition, exposure to harmful materials, unhealthy diet, physical injuries and underlying diseases. Physical injuries such as blunt force trauma can activate acute inflammatory responses that can lead to chronic inflammation. This highlights the need for effective therapies for many of these important diseases. Despite this urgent need for treatment of these types of diseases, the lack of effective therapy continues to be one of the greatest health challenges of our time.
A key goal of the Institute for Biomedical Therapeutics (IBT) is to pursue and exploit innovative new approaches to develop breakthrough therapy. By bringing together an integrated team of expert scientists, engineers, and biomedical researchers, the IBT is in a unique position to develop innovative solutions integrating the state of the art technologies in molecular therapeutics, pharmaceutical delivery, biomaterials, nanomedicine, and medical devices into a seamless paradigm-changing care for patients.
Towards this goal, the IBT has formed the NeuroRx team. This team will focus on the design and development of breakthrough therapies based on key molecular and pharmaceutical insights for each disease. The NeuroRx team will also establish effective means for their delivery to targeted sites by using suitable formulation, bioengineering techniques, and nanotechnology. Enabled by the multidisciplinary and closely collaborative team of IBT, these integrated systems will enhance selectivity and specificity to improve efficacy, while reducing the potential for adverse effects. The result of this strategy is a significantly enhanced overall outcome for the treatment of neurosensory ailments, while ensuring the safety and well being of the patient.
NeuroRX focuses on implementation of novel drug delivery systems and bioelectronic implants that may deliver drugs across the blood-brain barrier in a highly selective manner. Novel micro and nanoeletromechanical systems known as MEMs or NEMs have been developed to deliver established and novel drug platforms. The technology has been applied to MEMS based drug delivery systems. This innovative bioengineering invention along with other potential drug delivery systems will revolutionize the field of neuropharmaceuticals.
In developing new systems for “smart” drug delivery, NeuroRx utilize the substantial expertise and experience of our faculty members to develop systems that can be useful in a wide range of ocular diseases. Using principles of microelectromechanical systems (MEMS) engineering, we have designed and tested prototypes of a small, refillable, implantable ocular drug pump (mini-drug pump) on the benchtop and in preclinical testing. Over the next few years, long-term biocompatibility testing will be conducted with various pharmacological agents to determine the device’s ultimate potential for ocular drug delivery. However, given preclinical results, prospects appear excellent for treatment of currently refractory problems encountered in conditions such as dry and wet aged related macular degeneration (AMD), uveitis, glaucoma, traumatic brain injury and neurodegenerative diseases (neurosensory disorders).
Ongoing Projects
The NeuroRx team in engaged in a wide range of novel therapeutics for the treatment of neurosensory disorders. They have employed novel strategies to significantly advance the efficacy of the patients.
Brain Project as applied to Neurosensory Disorders
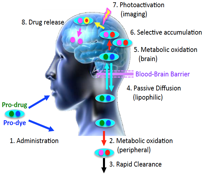
 Diseases that affect the brain such as stroke and trauma remain major causes of morbidity and mortality. Traumatic brain injury (TBI) alone occurs in more than 1.5 million Americans each year. In addition to being life threatening, many milder forms of TBI’s can lead to serious long-term cognitive and physical sequelae. NeuroRx is developing a paradigm changing nanocage-infrared-triggered (NIT) therapeutic system for use during the initial period or “golden hour(s)” after sustaining a TBI. The science used to engineer the NIT system will make it ergonomic and robust enabling first responders to use this system to limit insults initiated by TBI.
Diseases that affect the brain such as stroke and trauma remain major causes of morbidity and mortality. Traumatic brain injury (TBI) alone occurs in more than 1.5 million Americans each year. In addition to being life threatening, many milder forms of TBI’s can lead to serious long-term cognitive and physical sequelae. NeuroRx is developing a paradigm changing nanocage-infrared-triggered (NIT) therapeutic system for use during the initial period or “golden hour(s)” after sustaining a TBI. The science used to engineer the NIT system will make it ergonomic and robust enabling first responders to use this system to limit insults initiated by TBI.
The NeuroRx is developing a first-of-its kind preventative treatment system for TBI. The uniqueness of the NeuroRx approach is seamless integration of the advanced principles of nanotechnology, chemical biology, and neurophotonics into a minimally invasive therapeutics that can be used by first-responders at the site of the accident to treat and mitigate the damage from the TBI in the initial period after the injury “golden hour(s)”. Specifically, the NIT system enables an intravenous (IV) administered nanocage-drug complex to be uncaged only at the site of injury. The nanocage-drug complex gets across the damaged blood-retinal and blood-brain barrier into the area of brain injury and then is released by infrared light transmitted across the intact skull by a wearable time-gated LED emitter array contoured much like a swimmer’s cap to fit the skull (Figure above). The time-gated feature results in not releasing the nanocage-drug while traversing in blood vessels but only when is it sequestered in the injured brain area. Hence, this system is engineered such that it can treat, in a highly directed/localized manner, the site(s) of injury without the a priori need for CT or MRI imaging to identify the location of the injury.
Bioactive Lipids
In conjunction with developing drug delivery devices, NeuroRx is also designing new drug therapies for treatment of ocular diseases like wet AMD, which is a leading cause of blindness in western countries. Approximately 15 million individuals in the U.S. suffer from some form of AMD, where the prevalence is expected to double by 2020. AMD is classified as either atrophic (dry) or exudative (wet) AMD; dry AMD is characterized by progressive degeneration of RPE and photoreceptors, while wet AMD has abnormal new vessel formation (i.e., choroidal neovascularization (CNV)), which bleeds into the retina damaging the retina (see Figure 1). It is estimated that 1.6 million people have the progressive form of AMD where active blood vessel growth and/or blood vessel leakage is part of the clinical presentation.
Current treatments for wet AMD primarily focus on inhibiting a protein called vascular endothelial growth factor (VEGF) that promotes the formation of new and leaky blood vessels. The most commonly used 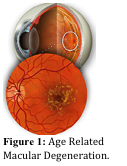 therapeutics include the biologic drugs LucentisTM, AvastinTM and EyleaTM, which must be administered via injections directly into the eyes every one or two months. Although these drugs are designed to bind to VEGF and limit its actions, they do not eliminate the source that produces VEGF. Additionally this type of therapy can be associated with a variety of side effects including stroke. Although these interventions are effective in addressing retinal edema and formation of new blood vessels, they have drawbacks due to the invasive intravitreal injections required on a regular basis. Moreover, the effectiveness of these therapies is limited to only one-third of AMD patients. This represents a major unmet therapeutic needs to develop new and more effective small molecule drugs that can better address the causes of this disease.
therapeutics include the biologic drugs LucentisTM, AvastinTM and EyleaTM, which must be administered via injections directly into the eyes every one or two months. Although these drugs are designed to bind to VEGF and limit its actions, they do not eliminate the source that produces VEGF. Additionally this type of therapy can be associated with a variety of side effects including stroke. Although these interventions are effective in addressing retinal edema and formation of new blood vessels, they have drawbacks due to the invasive intravitreal injections required on a regular basis. Moreover, the effectiveness of these therapies is limited to only one-third of AMD patients. This represents a major unmet therapeutic needs to develop new and more effective small molecule drugs that can better address the causes of this disease.
NeuroRx initiatives have focused on the deployment of novel anti-inflammatory agents for the treatment of several major diseases of the eye. This technology was based on the work of NeuroRx members, which provided insights into inflammation and mechanisms leading to tissue resolution. This insight has propelled the NeuroRx team to develop compounds that promote tissue restoration from damaged and inflamed state back to homeostasis. Compounds of this type can also inhibit the formation of new blood vessels, or angiogenesis, which is the source of a number of ophthalmic disorders such as AMD.
Mas Agonist
The renin angiotensin system (RAS) is most known for its ability to regulate blood pressure through the action of the first identified active peptide, angiotensin II. However it was discovered that other peptides of the RAS could accelerate wound healing and have regenerative properties. It was discovered that angiotensin (1-7) (A(1-7)), a metabolite of angiotensin II, is able to accelerate the regeneration of injured tissues, including bone marrow and skin. Members of the NeuroRx are leaders in exploring how to harness the protective arm of the RAS for the treatment of neurosensory, metabolic and ocular diseases. To this end, NeuroRx is developing novel formulations and delivery methods to enhance the patient adherence, which include nanoparticle and MEMs technology.
Next, read about our Research Faculty.